Fischer-Tropsch Processes Operate in the Mantle of the Earth
Carbon, Hydrogen, and Oxygen Reactions in Earth’s Mantle
Excerpt from my book The Truth About Energy, Global Warming, and Climate Change: Exposing Climate Lies in an Age of Disinformation
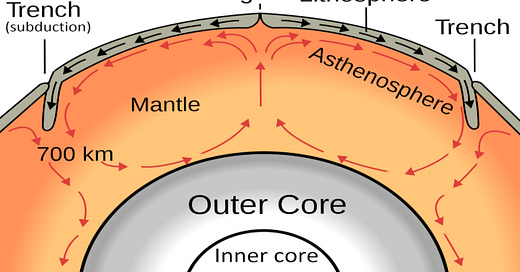
The mantle lies between the superheated core and the thin outer layer, the crust. It is about 2,900 km (1,802 miles) thick and makes up approximately 84 percent of Earth’s volume and 67 percent of its mass.1
A leading college textbook on structural geography describes this earth structure as containing two outer shells distinguished by their chemical composition and mineralogy. The text notes the following:
The upper shell, referred to as the crust, is made up of various sedimentary, metamorphic, and igneous rocks that are rich in silica, and are composed primarily of the minerals feldspar and quartz. The lower shell, called the mantle, is primarily peridotite, an igneous rock that is relatively poor in silica and is composed mostly of the minerals olivine and pyroxene. Due in large measure to the different densities of these common minerals, crustal rocks typically are less dense than mantle rocks. The oceanic crust is up to about 10 km thick and the continental crust is about 50 km thick, whereas the mantle is about 1,800 km thick.2
As we saw in the previous chapter, subduction zones are areas where a plate with oceanic crusts descends beneath a plate with continental crust. In the process of subduction, organic and inorganic carbon are both drawn into the mantle. A 2019 study published in Nature demonstrated that subducting carbon resides in the ocean in the form of carbonate shells and remains of marine organisms, as well as carbonite in the oceanic lithosphere. The lithosphere is one of the top layers of the earth, composed of the crust and the upper part of the mantle. Not all the subducted carbon in the mantle is recycled to Earth’s surface. Deeply subducted carbon potentially forms diamonds.3
A 2016 study published in Nature Geoscience studied boron isotopes in subducted carbonatites from forty million to 2.6 billion years ago. The study provided evidence for carbon of primordial origin to be in Earth’s mantle. But the study also found that the subduction rate has varied over geologic time. During the first two billion years or so, the mantle was much hotter than today, a phenomenon that prevented subduction plates from penetrating the mantle as deeply as today. During the last two billion years, a cooler mantle has allowed subduction plates to move at greater depths, possibly to Earth’s core-mantle border.4
Earth’s mantle also contains abundant hydrogen. One of the chemical processes that release hydrogen into the mantle is serpentinization. We also know that seawater percolates through tectonic fractures in subduction zones. Serpentinization occurs when this percolation water transforms ultramafic rocks into the crystal structure of the minerals found in the rock. Ultramafic rocks are high in magnesium and silica, such as igneous olivine and peridotite. Ultramafic rocks are igneous rocks abundant in the mantle composed of magnesium and silicon.
An example of the process is the serpentinization of peridotite into the mineral serpentine. Peridotite is an ultramafic igneous rock. Peridotite consists primarily of olivine and other iron- and magnesia-rich minerals (generally pyroxene).5 In serpentinization, water acts as a catalyst. The chemical reaction causes the iron and magnesium minerals in the ultramafic rocks to transform into serpentine-group minerals (e.g., antigorite, chrysotile, and lizardite). The chemical reaction involved in serpentinization releases hydrogen through a dehydroxylation process.6 Dehydroxylation consists of a heating process in which the hydroxyl group (OH) is released from the ocean water (H2O) involved in the chemical reaction.7
Another complexity IPCC adherents need to consider in comprehending the intricacy of Earth’s hydrogen cycle is discussed in a 2017 scientific study published in the Proceedings of the National Academy of Sciences. Using advanced diffraction tools, the scientific team found that hydrogen freed from goethite (FeO2H) can rise through Earth’s mantle to the surface.8 Goethite is a hydrous compound found in subduction slabs deep in the lower mantle. Subducted ocean water also acts as a catalyst in freeing hydrogen from goethite in the mantle.9
Oxygen is the most abundant element in Earth’s mantle. The top three elements in the mantle are oxygen (45 percent), magnesium (23 percent), and silicon (22 percent). Scientists from the University of Bonn in Germany demonstrated in laboratory studies that majorite, at a depth of several hundred kilometers underground, stores oxygen under high pressures and temperatures. Majorite is a granite-like mineral found in the mantle that acts as an oxygen reserve. The Bonn scientists also stressed that the majorite acts as an oxygen elevator. Professor Christian Ballhaus from the Mineralogical Institute at the University of Bonn emphasized the importance of majorite’s oxygen mechanism in the mantle to life on Earth. “According to our findings, planets below a certain size hardly have any chance of forming a stable atmosphere with a high water content,” explained Arno Rohrbach, a doctoral student at the Mineralogical Institute. “The pressure in their mantle is just not high enough to store sufficient oxygen in the rock and release it again to the surface.” Nearing Earth’s surface, the pressure in the mantle becomes too weak to maintain the majorite. As the majorite decomposes, the oxygen is released. The released oxygen bonds with hydrogen to form water. Ballhaus stressed that without this mechanism, Earth would not be known as the Blue Planet.10
Earlier in the chapter, we identified that gasification produced hydrogenated carbon monoxide in the Fischer-Tropsch process. The gasification chemical equation is C + H20 ® CO + H2. As just shown, hydrogen, carbon, and oxygen are all present in Earth’s mantle. So too, carbon monoxide is present.11 Geoscientists have established that there is possibly as much water in the mantle as in the oceans.12 Moreover, research published in 2013 found that deep in the mantle, at the high temperatures and pressures, the hydrogenation of carbon takes place relatively easily. Thus, all the chemical reactions, temperature, and pressure conditions necessary to make hydrocarbon fuels are present within the mantle.13
Earth’s mantle forces are also sufficient to cause hydrocarbon fuels to rise to the surface. Convection in the mantle is a process that occurs when materials near the core heat up and rise to the surface. Suppose a Fischer-Tropsch–like process occurs within the mantle of Earth. As hydrocarbons form and heat up, the process of convection could carry the deep-Earth abiotic hydrocarbons so formed to Earth’s surface. We now have the required conditions for abiotic oil created in the mantle to pass through tectonic fractures in the bedrock crust and pool in sedimentary rock at Earth’s surface.
In the process of migrating into sedimentary rock reservoirs, the abiotic hydrocarbons created in the mantle pick up organic biomarkers. The organic biomarkers in Earth’s abiotic oil have tricked Western petroleum geologists into thinking hydrocarbon fuels are organic in origin.14
“Mantle,” National Geographic, no date,. See also: Structure of the Earth’s interior,” GeologyScience.com, no date. See also: Jijo Sudarsan, “Interior of the Earth: Crust, Mantle, and Core,” ClearIAS.com, last updated on July 10, 2016.
David D. Pollard and Stephen J. Martel, Structural Geology: A Quantitative Introduction (Cambridge, U.K., and New York, NY: Cambridge University Press, 2020), p. 5.
Terry Plank and Craig E. Manning, “Subducting Carbon,” Nature, Volume 574 (October 16, 2019), pp. 343-352.
University of Notre Dame, “Key indicator of carbon sources in Earth’s mantle,” ScienceDaily, November 9, 2016, . The ScienceDaily article reported on the following study: Samuel R.W. Hulett, Antonio Simonetti, et al., “Recycling of subducted crustal components into carbonatite melts revealed by boron isotope,” Nature Geoscience, Volume 9 (November 7, 2016), pp. 904-908.
“Peridotite,” Encyclopedia Britannica, no date.
Alessandro Gualtieri, Carlotta Giacobbe, and Cecilia Viti, “The dehydroxylation of serpentine group minerals,” American Mineralogist, Volume 97, Number 4 (March 2012), pp. 666-680.
Enrico Bonatti, James R. Lawrence, and Noris Morandi, “Serpentinization of ocean peridotites: temperature dependency of mineralogy and boron content,” Earth and Planetary Science, Volume 70, Issue 1 (September 1984), pp. 88-94.
Qingyang Hu, Duck Young Kim, Jin Liu, et al., “Dehydrogenation of goethite in Earth’s deep lower mantle,” Proceedings of the National Academy of Sciences of the United States of America, Volume 114, Issue 7 (February 14, 2017), pp. 1498-1501, . See also: “Freeing Hydrogen in Earth’s Lower Mantle,” Carnegie Science, February 2, 2017.
Yanhao Lin and Wim van Westrenen, “Oxygen as a catalyst in the Earth’s interior?” National Science Review, Volume 8, Issue 4 (April 2021).
“Scientists: Deep Sea Mineral Acts as a Oxygen Reservoir, Stopping the Earth from Becoming a Barren Planet,” UnderwaterTimes News Service, September 26, 2007, . The scientific study can be found here: Arno Rohrbach, Chris Ballhaus, et al., “Metal saturation in the upper mantle,” Nature, Volume 449, Number 7161 (September 27, 2007), pp. 456-458.
Eglantine Boulard, Alexandre Gloter, Alexandre Corgne, et al., “New host for carbon in the deep Earth,” Proceedings of the National Academy of Sciences of the United States of America, Volume 108, Number 13 (March 29, 2011), pp. 5184-5187.
Andy Coghlan, “There’s as much water in Earth’s mantle as in all the oceans,” New Scientist, June 7, 2017. See also: Andy Coghlan, “Massive ‘ocean discovered towards Earth’s core,” New Scientist, June 12, 2014. The study in question is the following: Brandon Schmandt, Steven D. Jacobsen, et al., “Dehydration melting at the top of the lower mantle,” Science, Volume 344, Issue 6189 (June 13, 2014), pp. 1265-1268.
Shawn E. McGlynn, Jennifer B. Glass, Kristin Johnson-Finn, et al., “Hydrogenation reactions of carbon on Earth: Linking methane, margarine, and life,” American Mineralogist, Volume 105, Number 5 (April 29, 2020).
N.G. Holm, C. Oze, O. Mousis, et al., “Serpentinization and the Formation of H2 and CH4 on Celestial Bodies (Planets, Moons, Comets),” Astrobiology, Volume 15, Number 7 (July 1, 2015), pp. 587-600. See also: Céline Martin, Kennet E. Flores, Alberto Vitale-Brovarone, et al., “Deep mantle serpentinization in subduction zones: Insight from in situ B isotopes in slab and mantle wedge serpentinites,” Chemical Geology, Volume 545 (July 5, 2020). See also: “Serpentinization,” Oxford University Press, Encyclopedia.com, no date. See also: Steve Drury, “What’s happening at the core-mantel boundary?” WileyEarthPages.com, June 16, 2014. See also: Terry Colins Assoc., “Rewriting the textbook on fossil fuels: New technologies help unravel nature’s methane recipes,” American Association for the Advancement of Science, news release, April 22, 2019.